Silicon is the second most abundant element in earth’s crust. It was discovered in 1823 by Jöns Jacob Berzelius. Silicon has tremendous uses including manufacturing of ceramic, glass, synthetic polymers and is an essential part of integrated circuits.
The mass number is different for each different isotope of a chemical element. Silicon (Si) has 24 known isotopes, with mass numbers ranging from 22 to 45. How many grams Silicon in 1 mol? The answer is 28.0855. We assume you are converting between grams Silicon and mole. You can view more details on each measurement unit: molecular weight of Silicon or mol The molecular formula for Silicon is Si. The SI base unit for amount of substance is the mole. 1 grams Silicon is equal to 0.8872 mole. Si I Ground State 1s 2 2s 2 2p 6 3s 2 3p 2 3 P 0 Ionization energy 65747.76 cm-1 (8.15168 eV) Ref. MKMD94 Si II Ground State 1s 2 2s 2 2p 6 3s 2 3p 2 P° 1 / 2 Ionization energy 131838.14 cm-1 (16.34584 eV) Ref. MZ83-1 (16.34584 eV) Ref. The Silicon Crystal and Conversion of Solar Energy to Electricity. Although the silicon atom has 14 electrons, their natural orbital arrangement allows only the outer four of these to be given to, accepted from, or shared with other atoms. See more Silicon products. Silicon (atomic symbol: Si, atomic number: 14) is a Block P, Group 14, Period 3 element with an atomic weight of 28.085. The number of electrons in each of Silicon's shells is 2, 8, 4 and its electron configuration is Ne 3s 2 3p 2. The silicon atom has a radius of 111 pm and a Van der Waals radius of 210 pm.
History and Discovery
Compounds of silicon were used long before the discovery of silicon. Antoine Lavoisier (1787) tried reducing silica, an oxide of silicon, to isolate silicon but failed. Sir Humphry Davy, in 1808 named the element silicium but also failed to isolate the element. The element was given its present name, silicon, by Thomas Thomson in 1817. Gay Lussac and Thenard successfully prepared impure amorphous silicon in 1811 but they did not characterize it as a new element. In 1823, silicon was finally prepared in pure form by Jöns Jacob Berzelius and hence given credit for its discovery [1]. Crystalline form of silicon was prepared, 31 years later, by Deville in 1854.
Silicon
| Periodic Table Classification | Group 14 Period 3 |
|---|---|
| State at 20C | Solid |
| Color | Crystalline, reflective with bluish-tinged faces |
| Electron Configuration | [Ne] 3s2 3p2 |
| Electron Number | 14 |
| Proton Number | 14 |
| Electron Shell | 2, 8, 4 |
| Density | 2.33 g.cm-3 at 20°C |
| Atomic number | 14 |
| Atomic Mass | 28.09 g.mol -1 |
| Electronegativity according to Pauling | 1.90 |
Occurrence
Silicon is the second most abundant element present in the earth’s crust. It is the seventh most abundant element in the universe. Silicon is formed through the oxygen-burning process in stars. Silicon reacts with oxygen to make silicon dioxide or silicates. Silicate minerals make up over 90% of earth’s crust. Silicon is rarely found in pure form. Group of minerals composed of silicon and oxygen are named silica. Silica is mostly found in crystalline state. Silicon minerals make up 90% of the earth’s crust and it can be used industrially in its naturally occurring form which makes it cheap and easily available raw material.
Physical Characteristics
Silicon is a brittle and hard crystalline solid. It has blue-grey metallic lustre. Silicon, in comparison with neighbouring elements in the periodic table, is unreactive. The symbol for silicon is Si with atomic number 14. It has a very high melting and boiling point. At standard conditions silicon also makes a giant covalent structure like other group 14 elements of periodic table do.
Chemical Characteristics
At room temperature, pure silicon acts as an insulator. Silicon is a semiconductor at standard temperature and pressure. Silicon is inert in crystalline form at low temperatures. Its conductivity increases with high temperature. Silicon readily reacts with oxygen [2]. It reacts with air above 900-degree centigrade. Melted silicon becomes very reactive and has to be stored in unreactive, refractory material to avoid any chemical reaction.
Significance and Uses
- Silicon minerals are used as structural compounds for instance as clays, silica sand, building mortar, stucco and building stones.
- Silicon minerals are used in making concrete.
- Silica is used to make fire brick (refractory brick) which is used in lining of furnace.
- It is used in making whiteware ceramics such as soda lime glass and porcelain.
- Silica is used in making optical fibre which has vast uses in telecommunications and computer networking.
- It is used in making fibreglass and glass wool which are used for structural support and thermal insulation.
- Silicon is used in making mechanical seals and waterproofing.
- Waxes and high-temperature greases are made using silicon.
- For medical purposes, silicon is used in breast implants and contact lenses.
- Silicon is used in making superalloys.
- Silicon is used for making silicon wafers which has wide applications in the semiconductor industry.
- Silicon is also essential for human beings i.e. skin, nail, hair and bone density of human beings depends on the amount of silicon present.
- Synthetic polymers called silicones are produced using silicon.
- Solar cells, semiconductors detectors, transistors and other semiconductor devices used in computer industry are made using silicon.
- Silicon is a crucial part of integrated circuits (ICs) which have vital importance in our electronic appliances, for instance, computers and cell phones [5].
- Free silicon is used for casting of aluminium and steel refining industry.
Health Effects
Silicon Atomic And Mass Number
Silicon is slightly hazardous. If crystalline silica is inhaled, it may lead to lung disease such as asthma or inflammation in upper lobes of lungs. Exposure of elemental silicon can cause eye or skin irritation.
Isotopes of Silicon
Silicon has three stable isotopes; Si-28, Si-29 and Si-30. Of these three naturally occurring isotopes Si-28 is the most abundant as it is produced in stars as well as during nuclear fusion reaction. The remaining two isotopes of silicon form only 7% of the naturally occurring silicon. So far twenty radioisotopes of silicon have been characterized. Most of these radioisotopes have half-life of few seconds only. Unstable isotopes of silicon decay to form aluminium or phosphorus isotopes.
Silicon 29 Mass Number
REFERENCES
[1]. Weeks, Mary Elvira (1932). “The discovery of the elements: XII. Other elements isolated with the aid of potassium and sodium: beryllium, boron, silicon, and aluminum”. Journal of Chemical Education. 9 (8): 1386–1412.
[2]. Voronkov, M. G. (2007). “Silicon era”. Russian Journal of Applied Chemistry. 80 (12): 2190. doi:10.1134/S1070427207120397
[3]. Rahman, Atta-ur- (2008-09-24). “Silicon”. Studies in Natural Products Chemistry. 35. Bejeweled 3 free download mac. p. 856. ISBN 978-0-444-53181-0
[4]. Jugdaohsingh, R. (Mar–Apr 2007). “Silicon and bone health”. The Journal of Nutrition, Health and Aging. 11 (2): 99–110. PMC 2658806.
Mass Number Of Silicon 32
[5]. Cheung, Rebecca (2006). Silicon carbide microelectromechanical systems for harsh environments. Imperial College Press. p. 3. ISBN 978-1-86094-624-0
Other Periodic Table Elements
- Copernicium
Copernicium is an artificially produced element and was synthesized in 1996. It has many unstable…
- Tennessine
Tennessine is a synthetic element that was discovered in 2010. It is highly radioactive and…
- Antimony
Free music recording software for mac. Antimony is a chemical element with symbol Sb and atomic number 51. A lustrous gray…
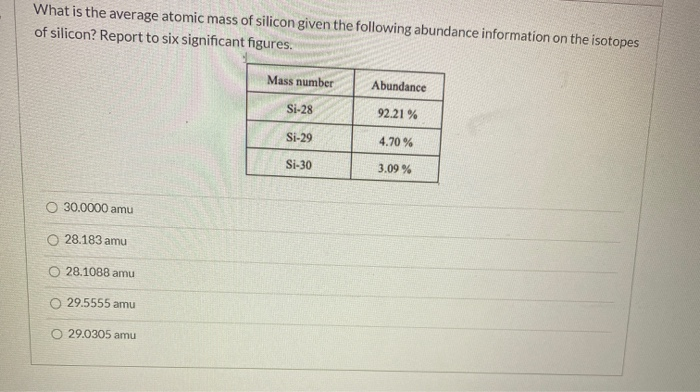
What is the average atomic mass of silicon?
Given the following data, calculate the average atomic mass of silicon.
isotope = Si-28
amu =27.9769
abundance(%) =92.18
isotope = Si-29
amu =28.9765
abundance(%) =4.71
isotope =Si-30
amu =29.9738
abundance(%) =3.12
Given the following data, calculate the average atomic mass of silicon.
isotope = Si-28
amu =27.9769
abundance(%) =92.18
isotope = Si-29
amu =28.9765
abundance(%) =4.71
isotope =Si-30
amu =29.9738
abundance(%) =3.12
1 Answer
Multiply the amu by the percentage of occurrence to arrive at an average atomic mass of

Explanation:
We take the amu of each isotope, multiply it by the percentage of occurrence, and end up with a weighted average:
This simplifies to:
Si Protons
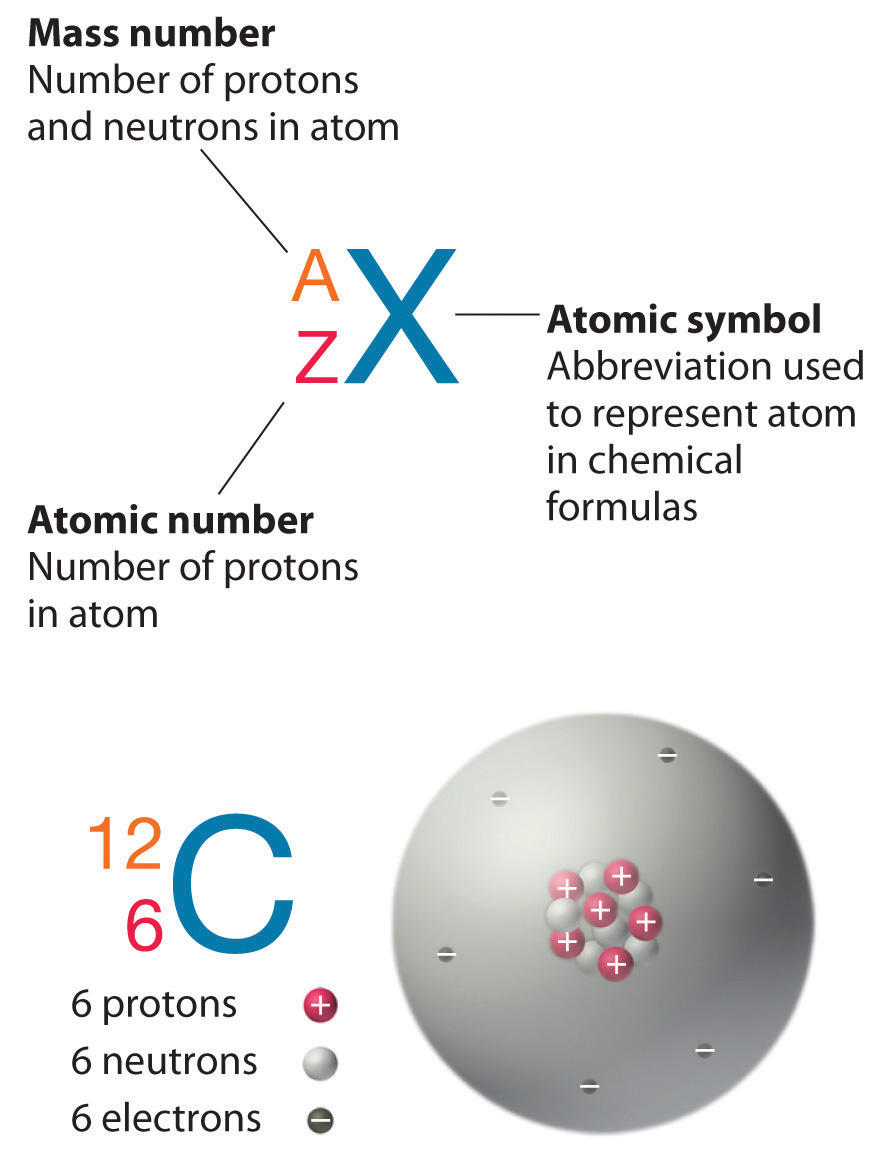
Checking 'the internet' to verify our answer, I found 28.0855 u ± 0.0003 u, so the answer calculated for our question is pretty close.
Related questions
